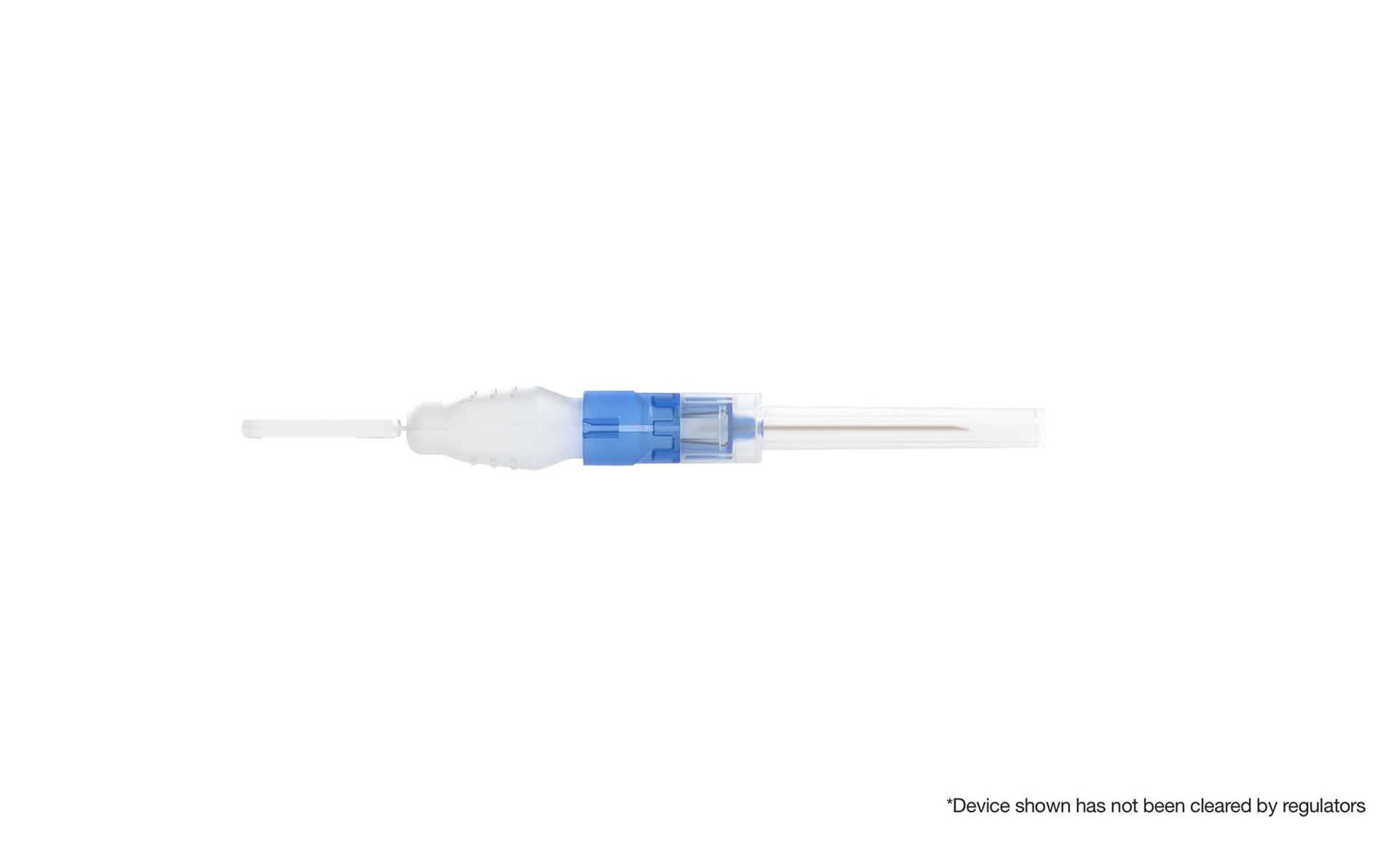
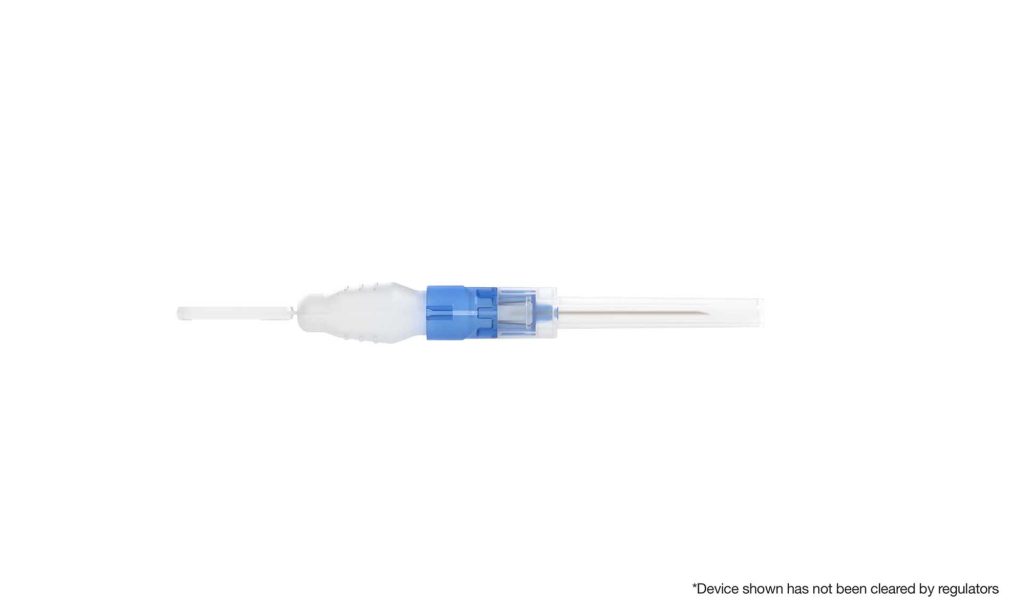
ApiJect is a medical technology company that has pioneered a new category of affordable prefilled injection devices. The technology was originally developed to help reduce the number of deaths due to syringe reuse and unsafe injection practices and solves important problems in emergency response and commercial pharma. Anchored by two well-known medical technologies – Blow-Fill-Seal (BFS) fill-finish and high precision injection molding – ApiJect’s novel drug delivery platform offers safety, cost, supply chain, and eco advantages over existing devices.
Interview with Jon Ellenthal, President at ApiJect.
Easy Engineering: What are the company’s main areas of activity?
Jon Ellenthal: We have created a technology platform for creating a new kind of prefilled drug delivery device, with broad applications across the universe of medical injections. Made from readily available pharmaceutical-grade plastic, ApiJect’s devices address global health concerns regarding injection safety by providing an affordable, easy-to-use alternative to glass vials for single-use injections.
Marc Koska founded the company because more than 1 million people around the world die each year due to syringe reuse and unsafe injection practices, especially in low- to middle-income countries. Prefilled BFS injection devices also can streamline and fortify the supply chain for commercial pharmaceutical companies and are more affordable than traditional prefilled syringes. That opens up a prefilled format option for more injections and potential competitive differentiation.
The Company’s manufacturing process uses readily available raw materials, operates on short lead times, and can scale rapidly, making ApiJect devices attractive to governmental organizations for medical countermeasures in the event of a bio emergency. Each Rommelag 460bp manufacturing line can produce more than 15 million BFS containers a month.
E.E: What’s the news about new products
J.E: BFS manufacturing has been proven for more than 50 years and is currently used to package more than 50 billion doses of sterile liquids every year. It’s a high-speed process with a simple supply chain, and the US Food and Drug Administration (FDA) recognizes BFS as an advanced aseptic process. Pharma-grade plastic resin offers a number of advantages over glass-based options, including cost-efficiency, eco-friendliness, and flexibility. But until ApiJect, no one had been able to adapt a BFS container for injections. ApiJect has figured out how to marry a soft plastic BFS container with a hard plastic needle hub to create a stable, high-performing prefilled injection device. In so doing, we have had to address considerations ranging from the quality of the seal and device activation to temperature management and squeeze force to consistent dose delivery to shipping and visual inspection. We have spent the last few years taking the device from a prototype to a full-scale commercial operating system.
E.E: What are the ranges of products
J.E: Our first prototype is a device suitable for up to a 1 ml dose of intramuscular injections. BFS containers and connectors support a variety of delivery shapes, sizes, and methods. Ultimately, we have built much more than a device. Rather we have created a technology platform for making prefilled drug delivery devices based on BFS technology. We have a deep device development pipeline and envision a large portfolio of devices that are suitable for the majority of medical injections.
E.E: At what stage is the market where you are currently active?
J.E: We are entirely focused on regulatory clearances and the commercial launch of the first device. We have begun the process with the FDA and are working with a drug partner on European Medicines Agency (EMA) clearances.
E.E: What can you tell us about market trends?
J.E: Driven by several factors, the global market for medical injections is projected to increase from 50 billion units this year to 70 billion by 2028—not including insulin. Demand for medical injections is driven by the increasing use of biologics, rising incidence of chronic diseases, an aging population, and the expansion of medical infrastructure. The prefilled injections sub-market is the fastest-growing segment. The ease and convenience of administration, reduction in dosing errors and drug misidentification, less drug waste, and the prospects of self-administration are all factors in the growing demand for prefilled injections.
E.E: What are the most innovative products marketed?
J.E: Prefilled devices are the best injection format, but they can be too expensive for many injections and overall supply is limited. We have created a new prefilled device that is both affordable and highly scalable. Our idea was deceptively simple, but our approach required a lot of innovation. We spent significant time developing and testing controls in the BFS process, vial design, wall thickness, mold dimensions, squeeze force, and other factors. We had to ensure that the right dose would be delivered. We had to innovate in the area of temperature management to protect temperature-sensitive molecules from the heat generated during the BFS filling process. This has resulted in a dramatic expansion of the drugs that can work with BFS. Finally, visual inspection is required for any drug that is injected into the human body. Incumbent automated systems are built for clear glass cylinders, but our device is neither transparent nor cylindrical, so we had to create a novel solution, which we did.
E.E: What estimates do you have for 2023?
J.E: We expect to apply for regulatory clearance in 2024. The market is showing interest in all three of our development areas—global health, commercial pharma, and emergency response— and we anticipate generating commercial revenues in 2025.