The risk of person-to-person viral transmission during the COVID-19 pandemic is especially relevant in manual, labour intensive and invasive processes such as packaging, labelling and inspection of products where close proximity among employees is difficult to avoid. Process automation can play a key role in risk reduction and help sustain the manufacture, testing and distribution of medications.
THE SUPPLY OF MEDICATIONS
The pharmaceutical industry has a great responsibility for supplying medications to patients who need treatment. The pharmaceutical supply chain is nevertheless a complex landscape. It involves sourcing of raw materials, manufacturing operations, distribution logistics and dispensing in many geographical regions by companies that range from large multinationals to medium and small size enterprises. A potential disruption in the supply of medications affects not only the balance sheets but more importantly, the health and safety of the population.
The supply of medications has recently been put to the test with the start of a global viral outbreak, namely the COVID-19 pandemic (1). There is a growing global demand for antivirals, sedatives and antibiotics needed to treat the symptoms of the disease, manage pain and prevent or control secondary infections (2,3). In addition, essential medications need to be available in the health system at all times to treat medical conditions in patients not related to the outbreak. However, employee absenteeism due to infections, suspected infections or fear of infections has already led to major drug shortages (4). Thus, the health authorities issued requests together with guidelines for the pharmaceutical industry to keep the production running (5–8). Temporary regulatory shortcuts include for example prolonging the expiry dates of medicines, extensions of the validity of GMP certificates that support manufacture and import of medicinal products, remote batch certification and audits, etc
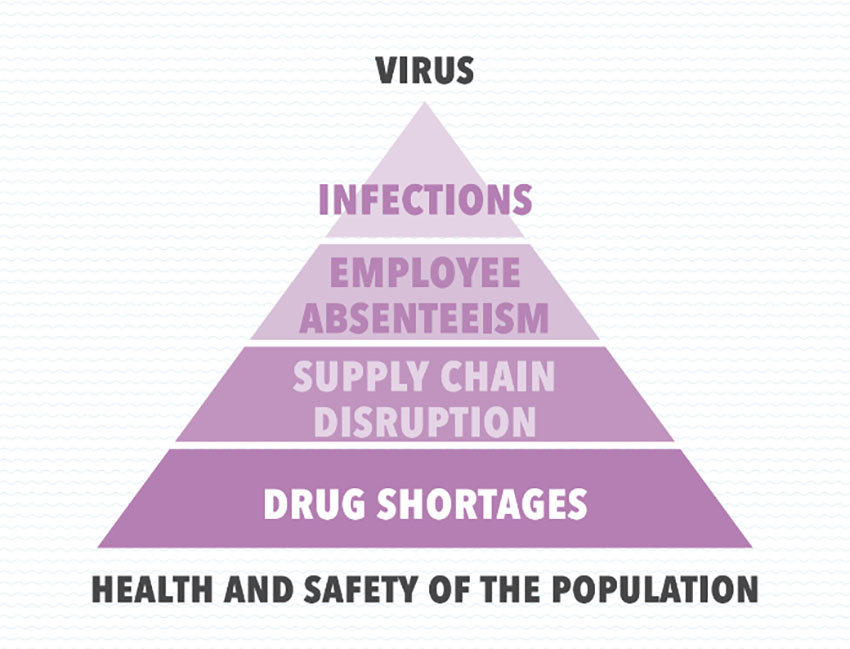
The SARS-CoV-2 virus can have an obvious escalating cascade effect on the health and safety of the population through a series of chain events starting with infections, suspected infections or fear of infections, followed by employee absenteeism and disruptions in the medicines supply chain and finally concluding with drug shortages.
RISK IDENTIFICATION AND MITIGATION
The question asked by the authorities is “How can potential risks be identified and mitigated in order to protect manufacturing facilities, employees, drug products and customers?”.
Risk identification posed by COVID-19 starts with understanding the epidemiology, infectivity and susceptibility to disinfection. The authors of (9) studied the currently available scientific literature related to properties of Coronaviruses in the context of minimizing possible disruption of the pharmaceutical supply chain. They identified person-to-person transmission of the virus as the greatest risk to the pharmaceutical supply chain (and thus to the community as a whole).
At the company level, mitigation efforts are connected to protecting the health and safety of employees (social distancing, working from home, screening, personal protective equipment, etc.) and the proper management of facilities and processes (viral clearance by heating, ventilation and air conditioning systems and HEPA filters) and in most cases have already been implemented.
At the process level, the risk of person-to-person viral transmission is especially relevant in manual, labour intensive and invasive processes such as packaging, labelling and inspection of products where close proximity among employees is difficult to avoid. Process automation can play a key role in risk reduction and help sustain the manufacture, testing and distribution of medications (Table 1).
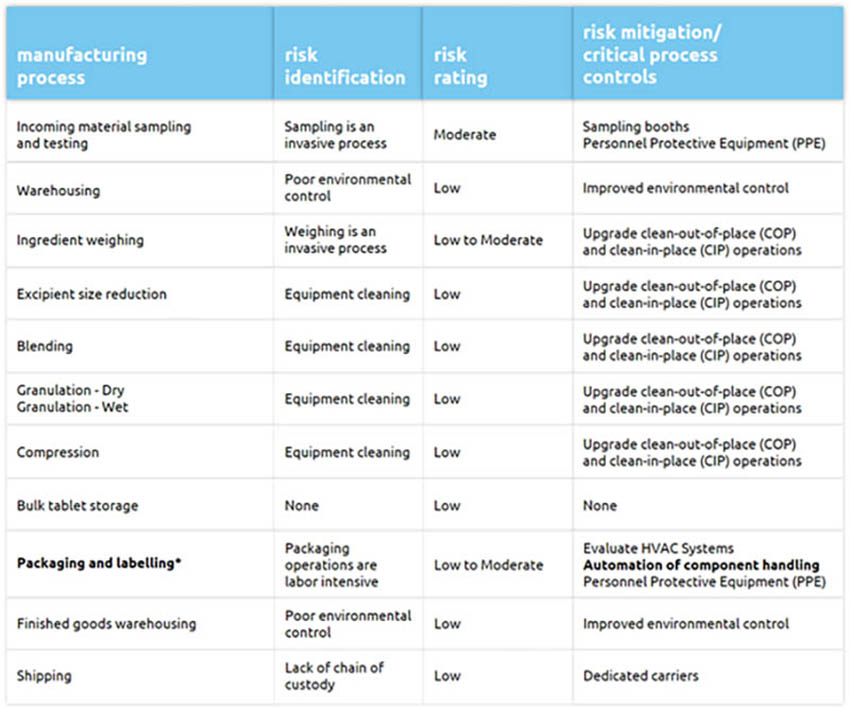
*Visual inspection of compressed tablets is mostly part of the packaging and labelling manufacturing process step. We believe same risks and risk mitigation efforts apply.
PROCESS AUTOMATION
Some of the most labour-intensive processes in pharmaceutical production are inspection campaigns of end products, such as tablets, capsules, softgels, injections, etc.
If performed fully manually, the operator needs to handle each product, examine it from different angles and decide, whether the product is defective. As a very labour-intensive activity, several dozen operators are needed in order to achieve the desired throughput. Moreover, the inspection by many different operators is prone to be subjective and unreliable.
When inspection is performed in a semi-automatic fashion, the operator’s hands are replaced by mechanical manipulators that handle the products but the inspection is still done manually. The process is faster and require less operators for the same throughput, however the issues with subjectivity and reliability remain together with the higher risk of potential viral infections among employees.
Fully automated solutions additionally use computer vision systems with digital cameras that replace human vision and computer algorithms that replace the human logic. Compared to manual inspection, automated inspection provides faster, more objective and more reliable performance even at long operating runs. Furthermore, it is non-invasive and it requires less human involvement, which not only reduces the risks of human-error, but also the risk of person-to-person virus transmission and thus the risk of shortages in drug supply due to absenteeism of employees. Read here about the risks of manual inspection and find here how precise and unbiased a machine vision system can be compared to human vision for quality control.
In Sensum, we specialise in the development and production of automatic visual inspection systems for the pharmaceutical and nutraceutical industry. Through its consistent innovation many leading multinational pharmaceutical companies (Pfizer, Novartis, Roche, Bayer, Eli Lilly, Teva, etc.) have recognised Sensum as the technology leader and a preferred supplier of machines for automatic visual inspection. Sensum provides high and mid capacity solutions for 100 % quality inspection of tablets, capsules, transparent capsules and softgels and in-line PAT solution for real-time visual monitoring of pharmaceutical processes.
To find out more about Sensum visual inspection systems visit the webpage, check youtube channel or follow on LinkedIn.

Inspection process is a process that has been identified as one of the most labour-intensive in pharmaceutical production of solid dosage forms. Sensum machines help pharmaceutical companies to achieve operational autonomy, allow operators to engage with higher added-value tasks and last but not least, help to reduce unnecessary human interaction.
Automation has been transforming the pharmaceutical industry towards achieving manufacturing excellence and reducing manufacturing costs. The recent events have highlighted an additional important role of automation: managing risks related to shortages of medicines during the COVID-19 pandemic, which helps keep the pharmaceutical production running.
References:
1. WHO announces COVID-19 outbreak a pandemic. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
2. Sheikh K. Essential Drug Supplies for Virus Patients Are Running Low. The New York Times. Available from: https://www.nytimes.com/2020/04/02/health/coronavirus-drug-shortages.html
3. Alexander GC, Qato DM. Ensuring Access to Medications in the US During the COVID-19 Pandemic. JAMA. 2020 Jul 7;324(1):31–2.
4. Research C for DE and. Drug Shortages. FDA. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/drug-shortages
5. Question & Answer – COVID-19 and WHO PQT Inspections | WHO – Prequalification of Medicines Programme. Available from: https://extranet.who.int/prequal/news/question-answer-covid-19-and-who-pqt-inspections
6. Research C for DE and. Planning for the Effects of High Absenteeism to Ensure Availability of Medically Necessary Drug Products. U.S. Food and Drug Administration. FDA. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/planning-effects-high-absenteeism-ensure-availability-medically-necessary-drug-products-0
7. MHRA regulatory flexibilities resulting from coronavirus (COVID-19). GOV.UK. Available from: https://www.gov.uk/guidance/mhra-regulatory-flexibilities-resulting-from-coronavirus-covid-19
8. Health C for D and R. Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency (Revised). U.S. Food and Drug Administration. FDA. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health
9. Cundell AM, Guilfoyle DE, Kreil TR, Sawant A. Controls to Minimize Disruption of the Pharmaceutical Supply Chain During the Covid-19 Pandemic. PDA J Pharm Sci Technol. Available from: https://journal.pda.org/content/early/2020/05/28/pdajpst.2020.012021

