Nectar is a medical device development company based in Southern California that focuses on improving patient outcomes through innovative medical technology. The company specializes in the design, development, and commercialization of medical devices that meet the highest standards of safety, efficacy, and quality.
Interview with Toska Ukaj, Scientific Content Writer at Nectar.
Easy Engineering: A brief description of the company and its activities.
Toska Ukaj: Our team of experienced engineers and medical professionals work together to develop cutting-edge medical devices that address unmet clinical needs. We are committed to delivering exceptional value to our customers by providing them with high-quality products that are easy to use, reliable, and affordable.
At Nectar, we take pride in our ability to bring novel medical technologies to market quickly and efficiently. Our state-of-the-art research and development facilities enable us to stay at the forefront of medical device innovation, and our commitment to quality ensures that our products meet or exceed all regulatory requirements.
E.E: What are the main areas of activity of the company?
T.U: Nectar is a multidisciplinary product development firm that specializes in end-to-end product design and development. We have expertise in mechanical engineering, software and firmware engineering, and industrial design. Our team is composed of experts in these disciplines, enabling us to deliver comprehensive solutions for our clients.
At Nectar, we place a strong emphasis on human factors and usability, ensuring that our products are designed with the end-user in mind. Our team has extensive experience in designing products that are intuitive, easy to use, and meet the needs of our clients’ target markets.

In addition to our core disciplines, we also have expertise in other areas, including electrical engineering, regulatory compliance (including FDA), and manufacturing. This enables us to offer a full range of services to our clients, from concept development to final product delivery. We take pride in our multidisciplinary approach, which allows us to deliver comprehensive solutions that meet our clients’ needs and exceed their expectations.
E.E: What’s the news about new products?
T.U: At Nectar, we are continuously working on a diverse range of exciting new projects. However, due to the competitive nature of our business and the confidentiality requirements of our clients, we can only provide a high-level overview of our ongoing efforts. For example, we are currently working on a home healthcare project that aims to provide relief to patients experiencing limb pain. Another project involves developing inspection equipment for a major healthcare provider’s production line. Additionally, we are working on a machine vision system for the aerospace industry that removes tough coatings and accurately inspects operations. We are also developing a projection system to correct vision during a youth’s eye development, with the aim of reducing the need for corrective lenses.
These are just a few examples of the innovative projects we are working on, with many more in active development for release to the market or for production use in the next 6 to 12 months. We are excited to bring these cutting-edge products to market and to continue pushing the boundaries of medical device design and innovation.
E.E: What are the ranges of products?
T.U: Nectar’s product portfolio includes several innovative medical devices designed to improve patient outcomes and address unmet clinical needs. Some of our products include:
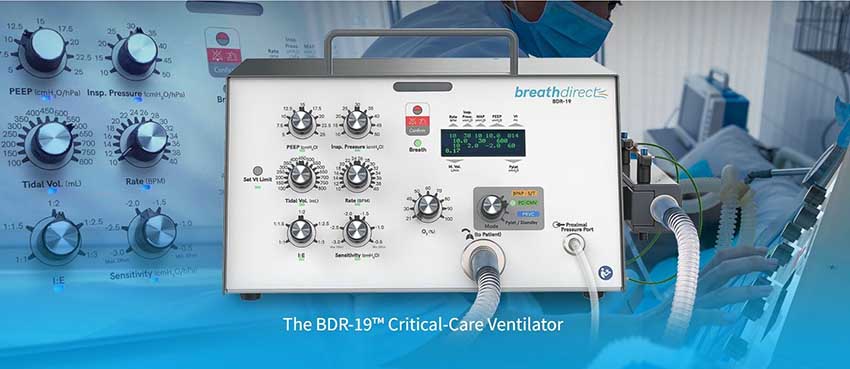
The BDR-19 Critical-Care Ventilator: This portable, full-featured, and affordable critical care ventilator is designed for ease of use and full spectrum support for patients in recovery or ICU. It features a durable battery and an intuitive user interface, making it an ideal choice for hospitals and healthcare facilities.

Limb Lengthener: Nectar partnered with Nuvasive to create a non-invasive limb lengthener device consisting of an intramedullary nail, locking screws, and reusable instruments. It is controlled by a user-centered, ergonomic, and intuitive External Remote Controller (ERC) for patients with Limb Length Discrepancy, offering an effective and non-invasive alternative to traditional limb lengthening methods.
Oxygenating Watering Systems: Nectar partnered with Bluemark Industries to develop the Pure Rain brand of oxygenating watering systems. These innovative home gardening products incorporate Bluemark’s water oxygenation technology, with a nanobubble generator integrated into 20 different SKUs. The products are designed to be innovative in performance, but familiar in usage, ergonomic, and with varying price points through a core chassis design.
E.E: At what stage is the market where you are currently active?
T.U: At Nectar, we primarily operate in the cutting-edge of the market, where both new and established clients are seeking to develop innovative technologies that challenge the status quo. We place a strong emphasis on user experience (UX) and usability, as well as technological development. Our clients are also deeply committed to technology and innovation, which fosters a collaborative environment where we can work together to create products that are not only groundbreaking but also user-focused.
By focusing on the leading and bleeding edge of the market, we are constantly pushing ourselves to stay ahead of the curve and explore new frontiers in medical device design. This approach allows us to work on exciting and groundbreaking projects that have the potential to transform the industry and improve patient outcomes. Overall, we’re dedicated to being at the forefront of technological innovation and design, and to collaborating with like-minded partners to bring bold new ideas to life.

E.E: What can you tell us about market trends?
T.U: In recent years, there has been a shift in the market towards a more personalized and home-centered environment. Products are becoming more geared towards individual or household use, particularly in the areas of home-healthcare and wellness. One example of this trend can be seen in diabetes care, where recent changes in reimbursements for Continuous Glucose Monitoring (CGM) have enabled patients to monitor their blood glucose levels from the comfort of their own homes. We believe that similar monitoring and wellness products will continue to emerge in the near future.
Another trend we’ve observed is the increasing pressure to monetize healthcare. This can be seen in rising healthcare premiums and medical expenses. However, we believe that the next big breakthrough will come from whoever can seamlessly connect healthcare databases with HIPAA compliance and connectivity from home to doctor, while still maintaining patient control. This will allow for a more streamlined and efficient healthcare system.
In the future, we envision a comprehensive wellness-centric system that connects exercise equipment, monitors for heart rate and other vital signs, and even data from smartphones and dietary tracking apps to a single healthcare database. Patients will be able to monitor and evaluate their health in real-time, and if they choose, share this information with their doctor. This will enable more proactive and personalized healthcare, as well as facilitate better communication between patients and healthcare providers.
E.E: What are the most innovative products marketed?
T.U: At Nectar, we’re proud to be at the forefront of medical device design and innovation. Our development process is unique and sets us apart from other companies in the industry. We start by identifying unmet needs in healthcare, then work closely with healthcare providers, patients, and other stakeholders to design and develop innovative medical devices that meet those needs.
One of our most innovative products is our BDR-19 critical-care ventilator, which was designed to revolutionize the way patients with respiratory conditions are treated. The device incorporates cutting-edge technology and features that make it more effective and user-friendly than any other product on the market. Throughout the development process, we worked closely with physicians and patients to ensure that the device met their needs and exceeded their expectations.
Our commitment to innovation and excellence is at the heart of everything we do. We’re constantly pushing the boundaries of medical device design and development, and we’re excited to continue leading the way in this exciting field.
E.E: What estimations do you have for 2023?
T.U: At Nectar, we’re thrilled to bring innovative products to market in the medical device and aerospace industries, in collaboration with our valued clients. We’re also excited to expand our manufacturing capabilities, including the production of Apple accessory products that meet cold-chain compliance requirements. Additionally, we’re currently working on a new project to deliver medicine deep into the lungs, which we believe will have a significant impact on patient care. As we look ahead to 2023, we can’t wait to continue pushing the boundaries of what’s possible in the healthcare industry and beyond. It’s sure to be an exhilarating and fulfilling year for us and our partners.