No matter what price you pay for a conveyor belt, its reliability and longevity will ultimately dictate its true cost. There are many things that determine their working lifetime such as surface abrasion, cutting & gouging, oil and heat. All of these are, of course, well known factors for which resistance should be engineered into a specifically designed rubber compound.
However, what is not well known are two other inescapable factors that have a huge influence on the operational lifetime of a rubber conveyor belt – ozone (O3) and ultraviolet light (UV). The damage they cause is extensive, occurs surprisingly quickly and certainly not limited to higher altitudes or sunny climates as some might think. Although it is relatively easy to make rubber resistant to the effects of ozone and ultraviolet, more than 90% of all conveyor belts sold in Europe have virtually no ozone and UV resistance. The deliberate absence of protection is designed to achieve greater price-competitiveness but the reality for the unsuspecting end-user is that it actually increases their costs. Here is how and why:
From protector to destroyer
One of the first design considerations that engineers should take into account when working with rubber are the effects of ozone. Almost all of the rubber used in conveyor belt manufacturing is synthetic. This is because different mechanical properties and characteristics can be specifically created depending on what the rubber will be used for and the working environments it will be subjected to.
Ozone (O3) occurs naturally in the upper atmosphere. It is formed continuously by the action of solar ultraviolet radiation on molecular oxygen (O2). At high altitude, ozone acts as a protective shield by absorbing harmful ultraviolet rays. Wind currents carry O3 to the atmosphere at the Earth’s surface. However, at low altitude, ozone becomes a pollutant. Ground level or “bad” ozone is not emitted directly into the air but is created by the photolysis of nitrogen dioxide (NO2) from sources such as automobile exhaust and industrial discharges. This is known as ozonolysis.
Ozonolysis
Ozonolysis is the reaction that occurs between the molecular structure (double bonds) and ozone.
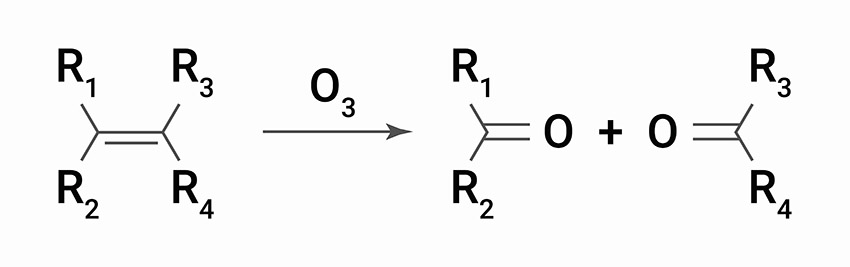
The scientific explanation is that the immediate result is formation of an ozonide, which then decomposes rapidly so that the double bond molecule is split. The critical step in the breakdown of molecular chains is when polymers are attacked. The strength of polymers depends on the chain molecular weight or degree of polymerization. The longer the chain length, the greater the mechanical strengths including the highly important tensile strength of the rubber. By splitting the chain, the molecular weight drops rapidly. There comes a point when very little strength remains and cracks starts to form. Further attacks occur inside the freshly exposed cracks, which continue to grow steadily until they complete a ‘circuit’ and the product separates or fails.
Exposure is inescapable because even tiny traces of ozone in the air will attack the molecular structure of rubber. It increases the acidity of carbon black surfaces with natural rubber, polybutadiene, styrene-butadiene rubber and nitrile rubber being the most sensitive to degradation. Although the first visible sign is when cracks start to appear in the surface of the rubber, depending on the level of ozone resistance that has been built into the rubber compound, the process of ozonolysis effectively begins when the conveyor belt leaves the production line.

Although the variability of weather, airflow patterns, seasonal changes, the level of emissions and climatic conditions domean that ozone concentrations can differ from one location to another, the fact is that ground level ozone pollution is ever-present and therefore its effects should therefore never be under-estimated.
A partner in crime
To make matters worse, ‘bad’ ozone has a partner in crime that also has a seriously detrimental effect on rubber. Ultraviolet light from sunlight and artificial (fluorescent) lighting accelerates rubber deterioration because it produces photochemical reactions that promote the oxidation of the rubber surface resulting in a loss in mechanical strength. This is known as ‘UV degradation’.

The rapid decline in the ozone layer in the upper atmosphere over the past several decades is allowing an increasing level of UV radiation to reach the earth’s surface. Continuous exposure is a more serious problem than intermittent exposure, since attack is dependent on the extent and duration of the exposure. Although the problem is worse in sunnier, hotter climates, it is a mistake to underestimate the damage that is caused because, as with ozone, the problem is ever-present even in the most moderate of environments.
The oxidation process
Ozone cracks form in rubber that is under tension. This is sometimes referred as ‘dry rotting’. It is important to bear in mind that the amount of tension (critical strain) needed is only very small. Even a belt that has not yet been fitted has a certain amount of intrinsic tension. The cracks are always oriented at right angles to the strain axis. The dynamic stress that a conveyor belt undergoes while in operation is considerable. Ozone attack occurs at the points where the strain is greatest.

The repeated action of the mechanical stress of the conveyor belt and the frictional process from the idler means that the rubber molecular chain will break to form what scientists refer to as a ‘free radical’. This triggers the oxidative chain reaction that forms a chemical process, which mechanically breaks the molecular chain and activates the oxidation process, which magnifies a whole range of problems such as the ability to resist abrasion.
Stress magnification
At first glance, having small cracks in the surface rubber may not seem to be a big problem but over a surprisingly short time, the rubber becomes increasingly brittle. As I have just mentioned, transversal cracks deepen under the repeated stress of passing over the pulleys and drums. The ozone continues to attack so the cracks will steadily grow until catastrophic failure occurs. Cracks often present other potential risks such as scrapers catching on them and tearing off parts of the cover. Re-splicing can also become increasingly difficult over time as the adhesion properties of the rubber diminish.

Yet another ‘hidden’ problem is that moisture seeps into the cracks. This then penetrates down to the actual carcass of the belt. In multi-ply belts, the fibres of the weft strands of the plies expand as they absorb moisture, which in turn causes sections of the carcass to contract (shorten) as the weft strands pull on the warp strands of the ply. This can result in tracking problems, which can be difficult to pinpoint, and which no amount of steering idler adjustment can compensate for. There can also be significant environmental and health and safety consequences because fine particles of dust penetrate the cracks and are then discharged (shaken out) on the return (underside) run of the belt.
Entirely preventable
As touched on earlier, ozone and ultraviolet damage is almost entirely preventable thanks to the use of modern technology. Several years ago, Netherlands-based Dunlop Conveyor Belting were among the very first in the world to make use of new technology that enabled the effects of ozone to be tested and measured. They equipped their laboratory with the very latest ozone testing equipment and introduced mandatory testing to EN ISO 1431 international standards for all of their rubber products.

As a direct result, special additives that act as highly efficient anti-ozonants and protect against the damaging effects of ozone and ultra violet became an essential ingredient in all of their rubber compound recipes without exception. Other leading European belt manufacturers, most notably Contitech in Germany, were quick to follow suit. Unfortunately for the end-user market in general, it still remains very rare for manufacturers located outside of Europe to use anti-oxidant additives.
EN ISO 1431 testing
To scientifically measure resistance to ozone in accordance with the EN ISO 1431 test method, samples are placed under tension (eg. 20% elongation) inside an ozone testing cabinet and exposed to highly concentrated levels of ozone for a period of up to 96 hours (@ 40°C, 50 pphm and 20% strain).

Samples are closely examined for evidence of cracking at two-hourly intervals and the results carefully measured and recorded. Experience has determined that in order for the rubber to be regarded as adequately resistant, the pass criteria needs to be that the rubber sample does not show any signs of cracking within the 96-hour period.
Worryingly, more than 90% of belts fail with the majority beginning to crack within 6 to 8 hours, especially belts imported from Southeast Asia whose strategy is to dominate the market by undercutting their competitors on price. Anti-ozonant additives are not cheap and are therefore simply not used.
Swept under the carpet
Despite its crucial importance, ozone and UV resistance is very rarely mentioned by belt manufacturers and suppliers and remains a subject that is swept under the carpet. A more sinister aspect is that for many belt suppliers, anything that prolongs the working life of belts is not good for business.

Because of their size, industrial conveyor belts are usually stored in the open air and can often be held in stock for long periods. During that time, they are vulnerable to the ever-present effects of ozone and UV radiation with anecdotal evidence of surface cracking being seen at the time of delivery.
No hiding place.
Ozone can be found everywhere. Even ‘normal’ air can have up to 0.01 ppm of ozone. The presence of electrical equipment or lighting can increase the ppm level to an even greater level. There is no hiding place. The importance of ozone and ultraviolet resistance can no longer be ignored. Unless conveyor operators start insisting on having belts that are ozone & UV resistant then they will continue to replace belts prematurely which had begun to deteriorate from the moment they are created.
For all buyers of rubber conveyor belts there should be two absolute pre-requisites when choosing any type of belt. Firstly, regardless of type, the rubber covers should have good resistance to abrasive wear. Just as importantly, they need to be fully resistant to the effects of ozone and ultraviolet. Without these essential properties the belt will not provide genuine value for money because it will need to be replaced far sooner than necessary. My advice is to always insist that the manufacturer/supplier provides certification confirming that the belt they are offering is fully resistant to ozone and UV in accordance with the EN ISO 1431 test method.

About the author
After spending 23 years in logistics management, Leslie David has specialised in conveyor belting for over 17 years. During that time, he has become one of the most published authors on conveyor belt technology in the world.

